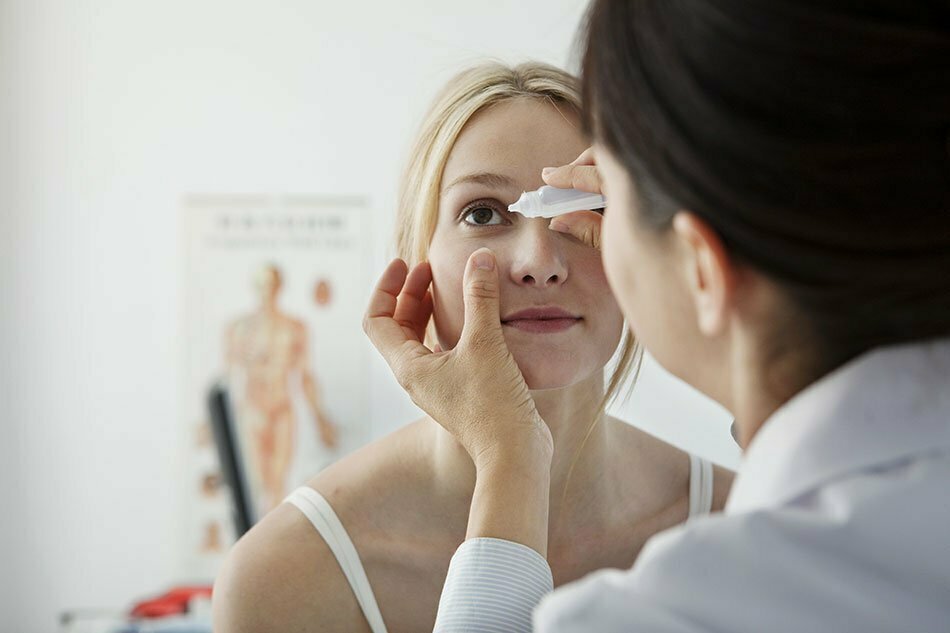
The FDA has approved the use of eye drops that could replace the need for reading glasses.
The newly approved eye drop, which has just hit the market, could change the lives of millions of older Americans with age-related blurred near vision, a condition affecting most people 40 and over.
Vuity, which was approved by the Food and Drug Administration in October, would potentially replace reading glasses for some of the 128 million Americans who have trouble seeing close-up. The new medicine takes effect in about 15 minutes, with one drop on each eye providing sharper vision for six to 10 hours, according to the company.
Toni Wright, one of the 750 participants in a clinical trial to test the drug, said she liked what she saw.
“It’s definitely a life-changer,” Wright told CBS News national correspondent Jericka Duncan.
Before the trial, the only way Wright could see things clearly was by keeping reading glasses everywhere — in her office, bathroom, kitchen, and car.
“I was in denial because to me, that was a sign of growing older, you know, needing to wear glasses,” she said.
It was in 2019 that her doctor told her about a new eye drop with the potential to correct her vision problems temporarily. The 54-year-old online retail consultant, who works from her farm in western Pennsylvania, instantly noticed a difference.
“I would not need my readers as much, especially on the computer, where I would always need to have them on,” she said.
Vuity is the first FDA-approved eye drop to treat age-related blurry near vision, also known as presbyopia. The prescription drug utilizes the eye’s natural ability to reduce its pupil size, said Dr. George Waring, the principal investigator for the trial.
“Reducing the pupil size expands the depth of field or the depth of focus, and that allows you to focus at different ranges naturally,” he said.
A 30-day supply of the drug will cost about $80 and works best in people 40 to 55 years old, a Vuity spokesperson said. Side effects detected in the three-month trial included headaches and red eyes, the company said.
“This is something that we anticipate will be well tolerated long term, but this will be evaluated and studied in a formal capacity,” Waring said.
Vuity is by no means a cure-all, and the maker does caution against using the drops when driving at night or performing activities in low-light conditions. The drops are for mild to intermediate cases and are less effective after age 65 as eyes age. Users may also have temporary difficulty in adjusting focus between objects near and far.
As of now, the drug is not covered by insurance. Doctors who spoke with CBS News said it’s unlikely that insurance will ever cover it because it’s not “medically necessary,” as glasses are still a less expensive alternative.
For Wright and millions just like her, the new drug is an easy backup solution — with a clear advantage.
“Just a convenience to have that option of putting the drops in and being able to go,” she said.